Journal of Advanced Biochemistry
PDGF Scientific Evaluation of the Different Grafting Materials and Complex Case Report with Vertical Resorption and Crestal Sinus Lift with 2s Implant
Maurizio Serafini1*![]() , Sara di Teodoro1, Luisa Romondio1, Luigi Zagaria2
, Sara di Teodoro1, Luisa Romondio1, Luigi Zagaria2
1Private Practitioner, Italy
2 School of Medicine University of “Aldo Moro” Bari, Italy
*Corresponding Author: Serafini M, Via Caduti sul Lavoro, 37 66100 CHIETI – Italy. E-mail: serafinidrmaurizio@gmail.com.
Citation: Serafini M, Teodoro S, Romondio L, Zagaria L. PDGF Scientific Evaluation of the Different Grafting Materials and Complex Case Report with Vertical Resorption and Crestal Sinus Lift with 2s Implant. Journal of Advanced Biochemistry. 2021;1(1):1-11.
Copyright: © 2021 Serafini M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received On: 17th March,2021 Accepted On: 3rd May,2021 Published On: 10th May,2021
Abstract
In dentistry, bone regeneration represents a crucial therapeutic aspect for a complete, balanced aesthetic and functional recovery. Over the years various techniques have been proposed and different types of materials (autologous and heterologous) have been subjects of critical reviews through clinical trials and histological examinations. The continuous research in optimum bone substitution materials has led to the introduction of many other materials in order to find the ideal one. The first intraoperative use of platelet-rich plasma (PRP) and it contribute to a faster bone healing was reported in 1998 by Marx et al. To demonstrate these effects, many studies followed to investigate the influence of PRP during bone augmentation. This case report shows a severe bone resorption successfully rehabilitated using animal bone graft and PRP which facilitates bone healing in several ways. PRP has a sticky texture which makes the graft easier to manipulate, it has procoagulant effects and it’s an important source of growth factors such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-b (TGF-b) 1 and 2 and many other cytokines involved in the angiogenic cascade. All these properties of PRP result as a boost in soft and hard tissue healing promotion, reducing bleeding risks and making patients postoperative outcome less painful and less expensive. In the present case, it was observed that PRP combined with animal bone resulted in significant, progressive and predictable clinical and radiographic bone regeneration. Further studies need to be done to fully understand long-term results of implant survival and success after the use of PRP.
Keywords: Bone regeneration, Bone grafting materials, Growth Factors, Severe bone defects, Osteogenesis, Osteoinduction, Osteoconduction, Dental Implant, Oral Rehabilitation, Platelet rich plasma, Vertical and Horizontal bone defects, PRP, PDGF.
Introduction
Bone grafting is a surgical procedure that replaces missing bone with material from patient’s own body, an artificial, synthetic or natural substitute. The biologic mechanism that provides a rationale for bone grafting are: Osteogenesis, Osteoinduction, Osteoconduction.
- Osteogenesis: the ability of an organic material to directly generate new bone formation from osteocompetent cells.
- Osteoinduction: process by which osteogenesis is induced providing a biological stimulus for induction, recruitment, stimulation and differentiation of undifferentiated mesenchymal cells into osteoblasts or chondroblasts. This mechanism depends on different factors, including the presence of specific proteins called Bone Morphogenetic Proteins (BMPs) usually found in the cortical bone [1-3].
- Osteoconduction: occurs when bone graft material serves as a scaffold for new bone growth, which is perpetuated by the native bone. Osteoblasts from the margin of the defect that is being grafted, utilize the bone graft material as a framework upon which to spread and generate new bone [4].
Classification of bone grafts based on material groups:
- Autologous bone grafts
- Homologous bone grafts
- Alloplastic grafts
Autologous bone grafts: bone is obtained from same individual receiving the graft. Bone can be harvested from nonessential bones, such as from iliac crest (endochondral) or mandibular symphysis and coronoid process (membranous) (Figure 1 and Figure 2).
Figure 1 Intraoral harvested bone Figure 2 Graft into the receiving site
Intraoral bone is a conspicuous source of autogenous bone, with the advantage of early revascularization, high BMP rate and high potential of vital cell, less risk of graft rejection. Disadvantage is that additional surgical site is required, another potential location for post-operative pain and complication and limited quantity of bone available.
Homologous bone graft: They derive from individuals of the same species, but harvested from an individual other than the one receiving the graft. They are obtained from cadavers duly treated and kept in bone banks. The advantages are remarkable because they can be found in great quantity and do not require any additional surgery; therefore, there are no surgical risks.
There are three types available:
- Frozen: Harvested – frozen – stored. The immunological reactions are known and in order to be reduced, blood type and cross-reactions have to be taken into account. Alternatively, it can be irradiated, but this procedure reduces the bone regenerative capacity.
- Freeze-dried (lyophilised): Harvested – freeze with different drying stages under hard vacuum, which destroys living cells and their osteogenic activity. It preserves the inorganic calcium/phosphorus matrix, but it is not largely used in dentistry because, in order for the regeneration to occur, it requires osteoclastic activity, which remodels the bone releasing BMPs and causes damages to the vital or host bone.
- Freeze-dried demineralized bone: it is mainly harvested from the skull and its size varies from 50/200 to 3000 micron. Particles under 200 microns are quickly reabsorbed, while the ones over 750 microns are reabsorbed very slowly and are defined as unabsorbable. Consequently, there is no BMP release, so the most widely used granulometry ranges from 200 to 750 micron.
The mineral content of the bone is degraded with solvents (ethanol, ether), washed in distilled water, and the inorganic part is removed using for 12/16 hours a demineralizing agent such as nitric acid or hydrochloric acid (0,6% concentration). This eliminates calcium/phosphorus and makes it sterile viruses and bacteria free. Then it is dehydrated and sterilized using ethylene oxide and/or irradiations.
Immunological reactions are practically non-existent.
Alloplastic bone grafts: There is a great number and variety of substitutes, re-absorbable and not. The most commonly used one is calcium sulphate (Figures 3-12).
class=”c2″>Figure 3 Severe bone dehiscence Figure 4 Implant inserted and calcium sulphate graft
Figure 5 Healing and loading Figure 6 Temporary prosthesis
Figure 7 Post extraction calcium sulphate graft Figure 8 Healing
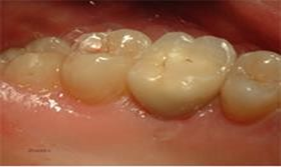
Figure 9 Implant insertion six months later Figure 10 Implant inserted
Figure 11 Definitive Crown Figure 12 Definitive Crown
Other alloplastic materials are Bio-glass, hydroxyapatite and animal bone [4-6]. All these materials are effective, safe and certified and are the most widely used because they are easily available on the market, contrary to homologous bone grafts. They come in different shapes – as mouldable blocks, rough or with cortical tissue grains, spongy and mixed. They serve as a matrix where, in a variable period of time depending on the material used, the material is absorbed by the cells (osteoclasts and osteoblasts) of the receiving individual [6]. In the re-absorbable ones (mainly animal-derived) a physiological remodelling occurs in a few months and they are replaced by physiological bone tissue. Follows a clinical case using P.R.P mixed with animal bone (Figures 13-17).
Figure 13 Root fracture and fistula Figure 14 Root fracture and fistula
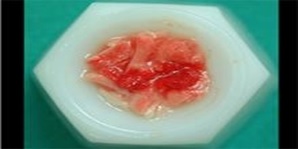
Figure 15 Animal Bone mixed with PRP Figure 16 Post extractive implant and Bone graft
Figure 17 Healing after eight months
The grafted bone heals in five stages [7]:
- Inflammation
- Revascularization (capillary buds invade the graft)
- Osteoinduction (differentiation of multipotent cells into osteoblasts)
- Osteoconduction (in-growth into the graft by means of the host)
- Remodeling
Case Report
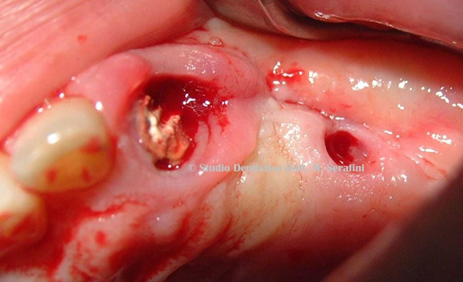
A 70 years old male patient with no significant medical history presented at our observation to evaluate the 35 years old prosthesis in her upper left region. Following the initial consultation, a CAT scan was taken to confirm clinical exam which showed bone loss around implant in position 2.6 and root fracture on tooth 2.3. (Figure 18 and Figure 19).
Figure 18 Clinical Situation
Figure 19 3D reconstruction shows severe bone loss
It was decided, accordingly with the patient, to rehabilitate the upper left maxilla in a two-stage surgery.
The patient was prescribed antibiotic therapy with Amoxicillin and Clavulanic Acid 1 gr. every 12 hours for 7 days, prior first-stage surgery, during which root extraction, surgical toilette and site reconstruction with bone regeneration was performed. Material used was animal bone mixed with PRP and Calcium gluconate in order to obtain a solid graft and a Biologic membrane. The area was sutured and the patient was given routine postoperative instructions (Figure 20-25).
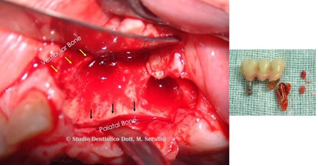
Figure 20 Yellow arrows point out Figure 21 PRP
Vestibolar Bone and Black Arrows Palatal Bone
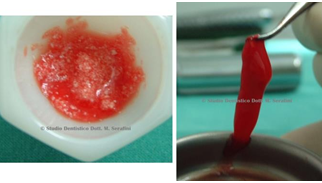
Figure 22 animal bone mixed with PRP and Figure 23 Bone Graft in-situ
Calcium Gluconate
Figure 24 Biologic Membrane in-Situ Figure 25 Suture
Healing appeared to proceed uneventfully and after ten months, CAT Scan Control was taken and second–stage surgery was planned (Figure 26 and Figure 27).
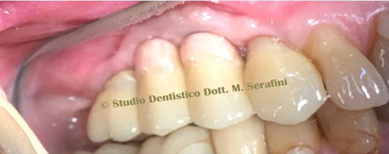
Figure 26 Cat Scan Control and Planning Figure 27 Soft tissue healing
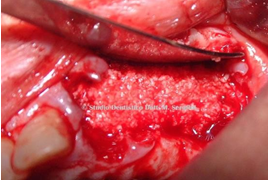
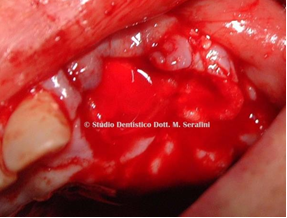
The treatment plan includes the insertion of three standard implants and one 2s Implant [8-11] implant dedicated for the Crestal sinus Lift. Second stage surgery was performed after antibiotic therapy with Amoxicillin and Clavulanic Acid 1 gr. every 12 hours for 7 days. Once the flap was detached, a very important amount of regenerated bone was found (Figure 28).
Figure 28 Bone tissue regenerated after graft.
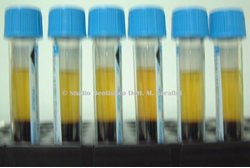
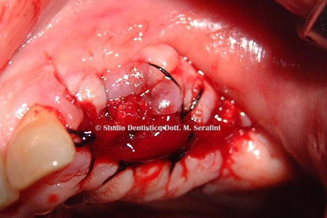
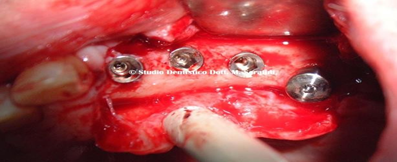
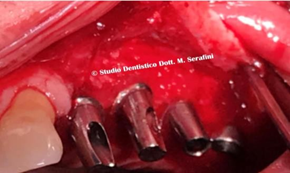
Venous blood sampling was taken, 4 tubes x 3, 5 ml with sodium citrate to obtain PRP (Figure 29 and Figure 30). Four implants (three standard and one 2s implant) were inserted. Vestibular bone was reinforced with autologous graft, collected during drilling, and protected by the biological membrane (Figures 31-39). in-situ
Figure 29 Venus blood, PRP, and Implant Figure 30 Biologic membrane and Autologous bone
in Its blister

Figure 31 Implants allocated, three with Figure 32 Autologous bone graft.
immediate loading and one (2s Implant) submerged.
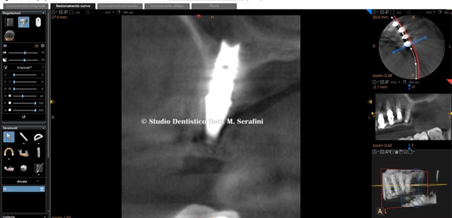
Figure 33 Biological Membrane in place Figure 34 Post Surgical X-Ray Control
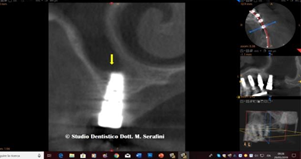
Figure 35 CAT Scan Control, the cut shows crestal sinus lift with the implant dedicated 2s Implant.
Figure 36 Definitive prosthetics in Zirconia-Ceramic
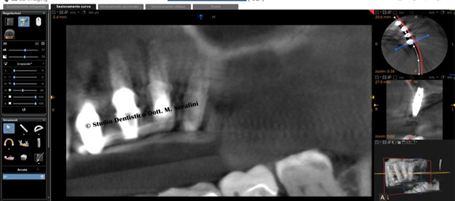
Figure 37 CAT scan Control 24 Months
Figure 38 Implants detail
Figure 39 Detail on 2s Implant.
Discussion
PRP utilize patient own blood, its preparation is easy, it doesn’t take more than 30 minutes and can be done while performing the surgery with no extra chair time. It is helpful in many surgical procedures and it is not harmful for the patient [12, 13]. As shown in the present case report and based on the author experience to date, the use of PRP in dental surgery gives good clinic and radiographic results in intra and post-operative bleeding control, graft stability, bone augmentation more rapid soft and hard tissue healing in both short- and long-term evaluation.
Conclusion
The scientific evidence regarding the efficacy and efficiency of PRP is still controversial, mostly on the long-term results. Within the limits of this study the author reports that the use of PRP improves the intra and post-operative healing and enhance bone augmentation thanks to growth factors. Given its availability and costs, it has become a very popular clinical tool in dentistry and it will change clinical practice more and more in several types of oral surgery.
References
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1998 Jun 1;85(6):638-46.
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proceedings of the National Academy of Sciences. 1986 Jun 1;83(12):4167-71.
- Buser D, Brägger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clinical oral implants research. 1990 Dec;1(1):22-32.
- Buser D, Dula K, Belser U, Hirt HP, Berthold H. Localized ridge augmentation using guided bone regeneration. I. Surgical procedure in the maxilla. International Journal of Periodontics & Restorative Dentistry. 1993 Feb 1;13(1).
- Simion M, Dahlin C, Trisi P, Piattelli A. Qualitative and quantitative comparative study on different filling materials used in bone tissue regeneration: a controlled clinical study. International Journal of Periodontics & Restorative Dentistry. 1994 Jun 1;14(3).
- Lazzara RJ. Immediate implant placement into extraction sites: surgical and restorative advantages. Int J Periodontics Restorative Dent. 1989; 9:332-43.
- Yazar S. Onlay bone grafts in head and neck reconstruction. InSeminars in plastic surgery 2010 Aug (Vol. 24, No. 3, p. 255). Thieme Medical Publishers.
- Kim J, Ha Y, Kang NH. Effects of growth factors from platelet-rich fibrin on the bone regeneration. Journal of Craniofacial Surgery. 2017 Jun 1;28(4):860-5.
- Shah R, Thomas R, Mehta DS. An Update on the Protocols and Biologic Actions of Platelet Rich Fibrin in Dentistry. The European journal of prosthodontics and restorative dentistry. 2017 Jun 1;25(2):64-72.
- Marx RE. Platelet-rich plasma: evidence to support its use. Journal of oral and maxillofacial surgery. 2004 Apr 1;62(4):489-96.
- Serafini M. New Concept of Maxillary Sinus Lift through a New Implant Dedicated: “2s Implant”. J Stem Cell Res Dev Ther. 2021; 7:061.
- Marx RE, Garg AK. Dental and craniofacial applications of platelet-rich plasma.
- Agrawal AA. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World journal of clinical cases. 2017 May 16;5(5):159.

 </span
</span
 <
<
 </span
</span
 <
<